Kremlin-owned media did not acknowledge Russia’s mistake after the application was submitted to the wrong online portal
Kremlin-owned media outlets were quick to promote a news story that the Russian Direct Investment Fund (RDIF) had successfully applied for European Union market authorization for the Sputnik V COVID-19 vaccine; the same outlets, however, were less eager to admit that the application was submitted via an online platform that the European Medicines Agency (EMA) has not used since 2015.
Pro-Kremlin sources have previously amplified negative coverage of foreign-made vaccines while promoting its own vaccine, Sputnik V. Kremlin-owned media also implied that the reason the EU (except Hungary) had not expressed interest toward Sputnik V vaccine was political bias, while the EU’s rationale was the lack of scientific evidence regarding the vaccine’s safety and efficacy, as the phase three trial results were not available until February 2, 2021. This case shows how the Kremlin tried to promote its vaccine while not correctly following the formal procedures imposed by the EU, while making excuses or ignoring its mistake. Public statements from Kiril Dmitriev, the head of RDIF, also implied that the Kremlin is prepared to label any rejection of Sputnik V vaccine in Europe as political.
The Sputnik V website promotes the vaccine as “The first registered vaccine against COVID-19.” On August 11, the vaccine was rushed through the approval process by the Russian Ministry of Health, after which Russian President Vladimir Putin publicly announced its successful development. The Ministry issued the vaccine’s registration certificate after it had reportedly been tested in just 76 people. The international scientific community expressed strong skepticism over the vaccine’s safety. Medical journal The Lancet published a report on Sputnik V’s phase 1 and phase 2 trials on September 4, 2020, and a report on the final phase 3 trial on February 2, 2021. The final report in The Lancet concluded that Sputnik V vaccine is safe, with an effectivity rate of 91.6 percent. On February 12, Hungary made the vaccine available to its citizens without waiting for the EU’s centralized approval. (Hungary has been a member of the European Union since May 1, 2004, and is therefore subject to its bureaucracy.) According to the official Sputnik V website, the vaccine is registered for use in 25 countries around the world.
Wrong submission portal
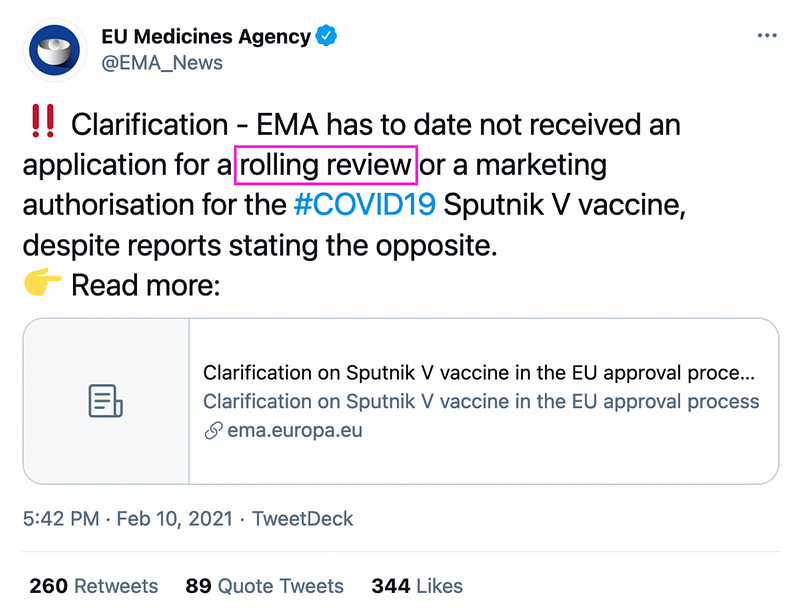
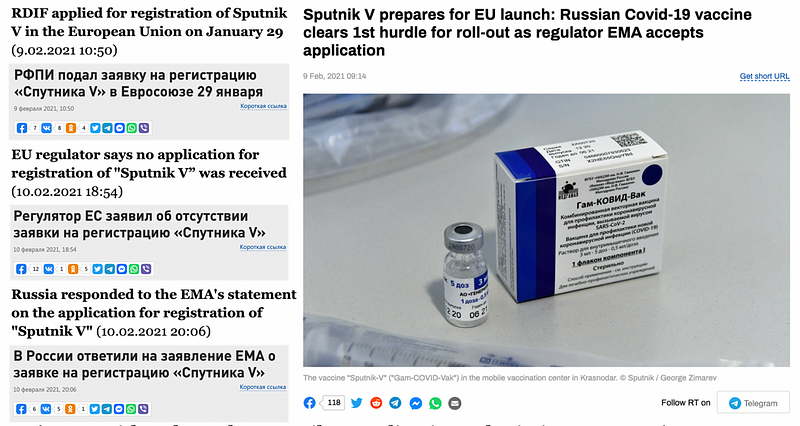
On February 9, RIA Novosti, a Kremlin-owned news agency, reported that RDIF, which financed the development of the Sputnik V vaccine, had applied for a “rolling review” with the goal of receiving marketing authorization for vaccine distribution in the EU and that it had received confirmation that application was received. A rolling review is an expedited process wherein EMA reviews data as it becomes available from ongoing studies. Once sufficient data is available, the company can submit a formal application for market authorization. This process is frequently used during public health emergencies.

On February 10, EMA, the health authority in EU that checks and recommends medicine for European Commission’s approval, denied that it had received the RDIF application. EMA promoted the clarification on Twitter.
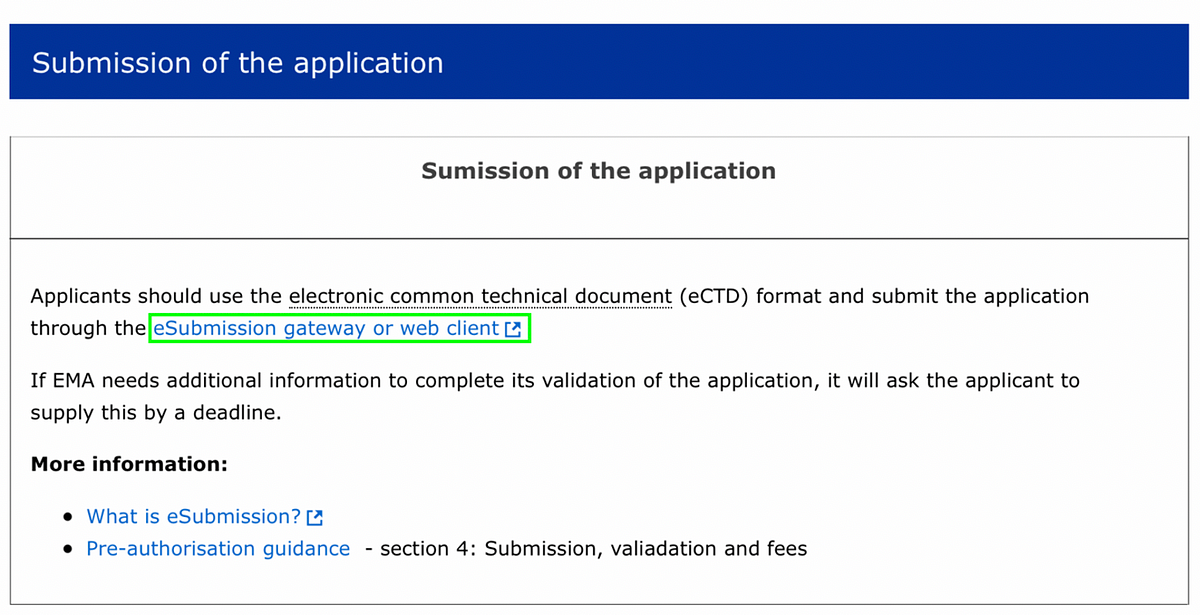
About an hour later, the Ministry of Health’s official Sputnik V account on Twitter published “evidence” of the application submission — a screenshot from the Common European Submission Portal.

The Common European Submission Platform has not been used as a part of the approval process for medicines since 2015.

The eSubmission Gateway is mentioned in the EMA’s step-by-step guidelines for obtaining an EU marketing authorization.
Kremlin-owned media ignores the mistake
Kremlin-owned media was quick to report on the initial announcement by the RDIF about its allegedly successful Sputnik V application, but it ignored the EMA’s clarification or did not acknowledge RDIF’s mistake.
For instance, on February 9, state-owned TV channel Rossiya 24 aired an announcement from Kiril Dmitriev, head of RDIF, who said in Russian:
Today the European regulator confirmed that the scientific consultation phase for Sputnik V has been successfully completed and that it is ready to review our application submitted on January 29, 2021. We hope that the review will happen soon, without political considerations. And of course, any major vaccine delivery to the EU can happen only when vaccination in Russia is completed, so it’s May and June this year.
The announcement was incorporated in the final news story, which aired at 8:00 p.m. Moscow time. In addition, the story emphasized how the vaccines produced in Western countries had allegedly been proven to be worse than Sputnik V. The story mentioned that the Pfizer-BioNTech vaccine in Germany did not prevent elderly people from being infected with COVID-19, and that none of the Western vaccines prevent the transmission of the new British and South African COVID-19 variants. Indeed, on February 8, 2021, German public media outlet DW reported that 14 residents of a nursing home in Belm tested positive for the new COVID-19 British variant after receiving two Pfizer-BioNTech vaccine doses. The story also claimed that Sputnik V produces more antibodies than the vaccine developed by RDIF’s British partners AstraZeneca and the University of Oxford. The accuracy of this claim is somewhat hard to discern, as The Lancet studies on the AstraZeneca and Sputnik V vaccines included antibody measurements but at different levels of granularity. Sputnik V is considered to have higher efficacy rate than AstraZeneca vaccine.
The next day, on February 10, the news broadcast at 8:00 p.m. Moscow time did not mention the confusion with the status of Sputnik V application for rollout review by EMA.
The confusion was reported by Kremlin-owned online media outlets likes RIA Novosti, RT, and Sputnik, but none of the reports acknowledged that RDIF submitted its application to the wrong platform. Instead, publications that covered EMA’s clarification put emphasis on the evidence published to the Sputnik V Twitter account that allegedly proves the EMA otherwise.
RIA Novosti published three stories based on what Dmitriev told Rossiya 24 on February 9, and only one story about the confusion with the Sputnik V application on February 10.

RT, the Kremlin’s international media outlet, published the news as it developed on its Russian version but had only one news story on its English version about the RDIF application for the rollout review process; none of these stories acknowledged the mistake.
Sputnik, the Kremlin-owned news outlet, has 29 language editions. The DFRLab identified that Sputnik’s editions in Azerbaijan, Belarus, the Czech Republic, Georgia, Kazakhstan, Kyrgyzstan, Latvia, Moldova, Uzbekistan, and Vietnam wrote only about the initial news that RDIF had “successfully” applied for Sputnik V authorization for the EU market. Sputnik’s editions for France, Greece, Iran, Poland, Serbia, and Turkey wrote only about the tweet to the Sputnik V account, posting a screenshot that allegedly proved that EMA received the application in response to EMA’s public announcement to the contrary. Sputnik Spanish, Italian and German editions covered both RDIF’s “successful” application and the subsequent confusion.

Some of Sputnik’s international editions covered the story rather voluntarily. For example, Sputnik Lithuania did not cover the story regarding the vaccine’s authorization in the EU but instead focused on a comment from Lithuanian Prime Minister Ingrida Šimonytė’s that the country would not use the Sputnik V vaccine. Sputnik Greece did not cover the initial story about RDIF allegedly successful application submission to EMA but published two separate stories about the following confusion — the first covered EMA’s statement that it had not received RDIF’s application; the second discussed the Sputnik V Twitter account’s response.
Nevertheless, most of Sputnik’s international editions published only the initial story about RDIF’s allegedly successful Sputnik V application submission to EMA or focused on the Sputnik V’s screenshot with application submitted on Common European Submission Portal, as the evidence that EMA’s public statement is wrong.
Nika Aleksejeva is a Digital Forensic Research Associate with the Digital Forensic Research Lab.
Follow along for more in-depth analysis from our #DigitalSherlocks.

